Atopic Dermatitis in a Child: A Review
Atopic Dermatitis in a Child: A Review
Epidemiology
Atopic dermatitis (AD) is
considered the third most prevalent skin disease worldwide affecting up to
2.79% of the population globally 1. The global burden of disease
caused by AD was the largest in high-income countries, female patients, and
children between 1-5 years 2. The prevalence of AD varies
a great deal between countries. It was reported that the prevalence of adult AD
was 4.9% of the population in the United States, 2.1% in Japan, and 20% of
children in Sweden 3,4. AD is a common, chronically
recurring skin inflammation with a benign course. However, disability-adjusted
life years (DALYs) showed a steep rise from 0.27% in 1990 to 0.36% in 2017 5. The detrimental effects of
AD encompass the academic, social, and occupational aspects of the individual’s
life. Moreover, AD is associated with other atopic conditions such as allergy 6 and asthma 7 and is associated with other
medical conditions including cardiovascular disorders 8, obesity 9, anxiety 10, and depression 11. Therefore, AD can be
regarded as a systemic illness with significant physical and psychological
manifestations 12.
Diagnosis
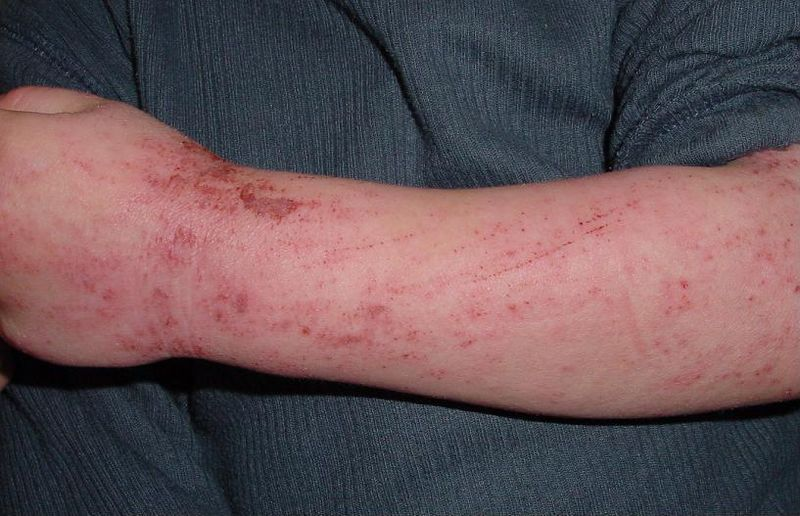
Atopic dermatitis is a chronic skin
disease characterized by skin pruritic inflammation that occurs mostly in
children. The onset of AD is usually between 3-6 months of age with most of the
patients (60%) developing skin eruption in the first year of life and about 90%
developing the disease by the age of 5 13,14. However, adults are also
affected. Relapse is a distinctive feature of the disease. Immunoglobulin E
(IgE) is often elevated in the sera of the patients 15,16. There is usually a positive
family history of type 1 allergies, allergic rhinitis, and asthma 7. The diagnosis of AD depends
on the clinical history of the patient, lesion morphology and distribution, and
the presence of positive family history. Hanifin and Rajka 17,18 and the United Kingdom (UK)
Working Party diagnostic criteria have been validated to be used for
epidemiological and population-based studies 19,20. In 2003, the American
Academy of Dermatology consensus conference suggested revised Hanifin and Rajka
criteria to apply to all age groups 21. On occasion, skin biopsy,
serum IgE, potassium hydroxide preparation, and patch testing can be carried
out to rule out other skin pathologies or associated skin conditions 22.
Food allergy, bacterial and viral infection
A food allergy may play a role in
the onset of AD skin symptoms, which can exacerbate disease severity and affect
treatment response 23,24. However, skin testing, in
vitro serum assays for allergen-specific IgE, and food restriction are not
generally recommended because of the uncertainty of their validity 25. Moreover, colonization with Staphylococcus
aureus impacts more than 90% of patients with AD aggravating the clinical
condition 26. Therefore, severe cases of
AD may need the addition of antibiotics even in the absence of obvious signs of
infection 27. In addition, viral infection
was detected in patients with AD mainly herpes simplex virus (HSV-1 or -2)
leading to eczema herpeticum and coxsackie A6 causing eczema coxsackium. Eczema
herpeticum causes fever, lymphadenopathy, keratoconjunctivitis, and multi-organ
damage leading to fatal outcomes 28. On the other hand, eczema
coxsackium may cause eruptions at the sites of AD lesions 29. Moreover, attention should
be paid to patients who received small box vaccination or were in contact with small
box-vaccinated persons because those patients with AD may suffer from a
potentially fatal condition, eczema vaccinatum 30.
Management
Managing AD
in children is challenging, involving a diversity of medications, behavioral
adjustments, and lifestyle modifications. Primary healthcare providers
including pediatricians and general practitioners are on the frontline of
diagnosing and treating AD in children, especially in rural areas where
specialist dermatologists or pediatricians may be unavailable 31,32. Therefore, the approach to
the diagnosis and management of AD by healthcare providers should follow the
updated guidelines of the American Academy of Dermatology (AAD) 22,33 and the American Academy of
Pediatrics 34. Knowledge and awareness of
the appropriate management of pediatric AD including topical corticosteroids
(TCS), emollients, water baths, and antibiotic therapies should be ensured
among the healthcare providers.
Patient and caregiver education
On the
other hand, extensive patient or caregiver education is of paramount importance
35. Not only information about
the clinical features and the natural history of AD should be given, but also
clear instructions about the given medications and the possible benefits and
side effects as well as the treatment plan according to the severity and
associated morbidity. The caregiver should understand that AD treatment is
individualized according to the underlying pathology and possible
co-morbidities 14.
Treatment
Topical emollients and water baths
Atopic
dermatitis is thought to relate to abnormalities in the gene encoding filaggrin
and the subsequent effect on the pro-inflammatory cascade. Therefore, the most
important variable in treatment is controlling trans-epidermal water loss 36. This can be accomplished
with the generous use of topical emollients to dry skin, especially when
applied after a shower or bath for 5-10 min in lukewarm plain water. However,
there is a dearth of conclusive evidence for optimal bathing duration and
ingredients to be used 37. An occlusive ointment should
be applied immediately after the water bath, soak-and-seal 38. In addition, all patients
with AD should utilize emollients, and thicker moisturizing treatments like
ointments and creams should be applied frequently. Bathing, showering, and
washing are important for skin cleaning to remove the adhering sebum, sweat,
and pathogens colonization (Staphylococcus aureus) 39. Moisturizing agents are
significant in preventing skin dryness. Regular use of moisturizers is
considered the cornerstone in the treatment of
AD. Moisturizers contain admixtures of ceramides, urea, lactate, and
salicylic acid that hydrate and repair the stratum corneum, thus mitigating
skin dryness, itching, flares, and infections 40,41. Ceramides constitute 50% of
stratum corneum lipids. Therefore, ceramides are considered a potential
therapeutic modality for AD 42. Several other moisturizers
have been searched with potentially promising results 43.
There is no evidence from the literature that soap and
detergents are effective in AD. Therefore, the use of soap should be limited to
a minimum in severe cases, during hot seasons when the skin is more liable to
dryness, and in sensitive patients 39. However, AD patients with a
positive culture for S. aureus showed improvement in disease severity after
using an antibacterial soap containing 1.5 percent triclocarban 44.
Topical corticosteroids
The
prescription of TCS is common among pediatricians with AD and primary care
providers 45,46 for cases with AD. The
utilization of TCS in the management of AD was approved by the Food and Drug Administration
(FDA) for inflammatory and pruritic skin conditions in 1955 47,48. The mechanism of action of
TCS is not clearly understood, however, TCS has a potential effect on the local
immune system and the function of the skin barrier 49. It has been reported that
TCS mitigates pruritis, inflammation, and S. aureus colonization in
children 50. Based on recent guidelines,
low-to-mid potency TCS is the first line for the management of AD for a short
period, less than 4 weeks 51. However, several factors
determine the choice of the TCS potency including case severity, the extent of
skin lesion, and the site of the flare 52. A recent systematic review
found no harm in the long use of TCS in patients with AD when TCS is used
intermittently “as required” or as “weekend therapy” to treat and prevent
flares 53. However, moderate-to-severe
AD cases may need long-term daily TCS therapy. A few studies reported that TCS
can be safely used by pediatricians for children with AD younger than 2 years 54,55. Patient desire,
pharmaceutical availability, cost, and a balanced assessment of benefits and
hazards all play a role in determining the maintenance therapy regimen and
product selection. Factors such as preference, availability of drugs, cost, and
a thorough assessment of benefits vs side effects should be taken into account
when deciding on a regimen and product for maintenance therapy. Step-wise
withdrawal of TCS should be applied when mid-to-high potency of TCS is used.
Moreover, treatment plans involving TCS should be discussed with the patients
or the caregiver of the child for better compliance and to eliminate the fear
of corticosteroid use 14,49.
Certain adverse effects should be considered when TCS is
used for children. Atrophy-related side effect of TCS was reported including striae
and purpura, hypopigmentation, focal hypertrichosis, telangiectasia, and
perioral dermatitis 56,57. Long-term TCS use was
reported to be associated with subclinical barrier disruption leading to
rebound flares when the drug is discontinued abruptly 58.
Antibiotics
Antibiotics are used as adjunctive therapy in patients with
AD who have S aureus colonization. The use of antibiotics should be guided by
anti-microbial sensitivity tests to ensure efficacy against resistant bacteria.
Bleach baths have been reported to be beneficial in the treatment of patients
with AD from the age of 6 months to 17 years of age and associated with
hyper-IgE syndromes 59,60. Moreover, numerous
compounds, many of which were not even labeled as antibiotics, exhibited
antibiotic capabilities. Several ingredient combinations have been identified
that may be useful in positively influencing the proliferation of the health
microbiome over disease-associated strains. For example, the combination of
colophonium and citral (known in the market as lemon myrtle oil and pine tar) 61. Skin microbiome shows
protective potentials against skin pathogens. Therefore, dysbiosis of the skin
microbiome can cause or trigger skin pathologies. A recent research study
confirmed that the reintroduction of coagulase-negative Staphylococcus strains
to human skin afflicted with AD led to a reduction of S. aureus colonization 62. The protective effect of the
coagulase-negative Staphylococcus strains (e.g., Staphylococcus epidermidis)
is mediated by the restoration of antimicrobial peptides that are essential
immune defense molecules 63,64.
Conclusion
Atopic dermatitis is a cumbersome skin disease affecting
children and young adults. Several triggering factors initiates the disease and
modulate severity. The treatment of AD is complex and multifaceted. Therefore,
primary care providers should have adequate knowledge and awareness to
diagnose, identify risk factors, and treat AD before referral to a specialist.
Several medications have been proposed as potentially effective in the
management of AD including moisturizing agents, baths, TCS, and antibiotics.
The choice of treatment regimen depends on the age, severity, distribution,
site of infections, and drug tolerability. Consequently, further research is
needed to set a protocol for the appropriate treatment of AD.
References
1. Mehrmal, S., Uppal, P., Giesey, R. L.
& Delost, G. R. Identifying the prevalence and disability-adjusted life
years of the most common dermatoses worldwide. Journal of the American
Academy of Dermatology 82, 258–259 (2020).
2. Urban,
K. et al. The global, regional, and national burden of atopic dermatitis
in 195 countries and territories: An ecological study from the Global Burden of
Disease Study 2017. JAAD International 2, 12–18 (2021).
3. Asher,
M. I. et al. Worldwide time trends in the prevalence of symptoms of
asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One
and Three repeat multicountry cross-sectional surveys. The Lancet 368,
733–743 (2006).
4. Barbarot,
S. et al. Epidemiology of atopic dermatitis in adults: Results from an
international survey. Allergy 73, 1284–1293 (2018).
5. Findings
from the Global Burden of Disease Study 2017. Institute for Health Metrics
and Evaluation
https://www.healthdata.org/policy-report/findings-global-burden-disease-study-2017
(2019).
6. Yin
Leung, A. S. et al. Quality and consistency of clinical practice
guidelines on the prevention of food allergy and atopic dermatitis: Systematic
review protocol. World Allergy Organ J 15, 100679 (2022).
7. Choi,
H. G. et al. Incidence of Asthma, Atopic Dermatitis, and Allergic
Rhinitis in Korean Adults before and during the COVID-19 Pandemic Using Data
from the Korea National Health and Nutrition Examination Survey. Int J
Environ Res Public Health 19, 14274 (2022).
8. Wu, J.
J. et al. The Use of Real-World Data to Evaluate the Association Between
Atopic Dermatitis and Cardiovascular Disease: A Retrospective Claims Analysis. Dermatol
Ther (Heidelb) 11, 1707–1715 (2021).
9. Guo,
Z., Yang, Y., Liao, Y., Shi, Y. & Zhang, L. Emerging Roles of Adipose Tissue
in the Pathogenesis of Psoriasis and Atopic Dermatitis in Obesity. JID Innov
2, 100064 (2021).
10. Hsu,
C.-J. et al. Correlation between anxiety and depression risk and atopic
dermatitis severity in Taiwan: A cross-sectional study. JAAD Int 7,
22–30 (2022).
11. Su, W. et
al. Anxiety, depression and associated factors among caretakers of children
with atopic dermatitis. Ann Gen Psychiatry 21, 12 (2022).
12. Brunner,
P. M. et al. Increasing Comorbidities Suggest that Atopic
Dermatitis Is a Systemic Disorder. Journal of Investigative
Dermatology 137, 18–25 (2017).
13. Bylund,
S., Kobyletzki, L. B. V., Svalstedt, M. & Svensson, Å. Prevalence and
Incidence of Atopic Dermatitis: A Systematic Review. Acta
Dermato-Venereologica 100, (2020).
14. Lyons,
J. J., Milner, J. D. & Stone, K. D. Atopic dermatitis in children: clinical
features, pathophysiology, and treatment. Immunol Allergy Clin North Am 35,
161–183 (2015).
15. Jeziorkowska,
R., Rożalski, M., Skowroński, K. & Samochocki, Z. Can evaluation of
specific immunoglobulin E serum concentrations of antibodies to aeroallergens
in atopic dermatitis patients replace skin prick tests method in clinical
practice? Postepy Dermatol Alergol 36, 478–484 (2019).
16. Wollenberg,
A., Thomsen, S. F., Lacour, J.-P., Jaumont, X. & Lazarewicz, S. Targeting
immunoglobulin E in atopic dermatitis: A review of the existing evidence. World
Allergy Organ J 14, 100519 (2021).
17. Hanifin,
J. & Rajka, G. Diagnostic features of atopic dermatitis. Acta
Dermatovener (Stockholm) Suppl. 92, 44–47 (1980).
18. Rudzki,
E. et al. Frequency and Significance of the Major and Minor Features of
Hanifin and Rajka among Patients with Atopic Dermatitis. DRM 189,
41–46 (1994).
19. Diepgen,
T. L., Sauerbrei, W. & Fartasch, M. Development and validation of
diagnostic scores for atopic dermatitis incorporating criteria of data quality
and practical usefulness. Journal of Clinical Epidemiology 49,
1031–1038 (1996).
20. Haileamlak,
A. et al. Validation of the International Study of Asthma and Allergies
in Children (ISAAC) and U.K. criteria for atopic eczema in Ethiopian children. British
Journal of Dermatology 152, 735–741 (2005).
21. Eichenfield,
L. F., Hanifin, J. M., Luger, T. A., Stevens, S. R. & Pride, H. B.
Consensus conference on pediatric atopic dermatitis* *A list of participants in
the Consensus Conference on Pediatric Atopic Dermatitis may be found at the end
of this article. Journal of the American Academy of Dermatology 49,
1088–1095 (2003).
22. Eichenfield,
L. F. et al. Guidelines of care for the management of atopic dermatitis:
section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol
70, 338–351 (2014).
23. Cartledge,
N. & Chan, S. Atopic Dermatitis and Food Allergy: A Paediatric Approach. Curr
Pediatr Rev 14, 171–179 (2018).
24. Schneider,
L. et al. Atopic dermatitis: a practice parameter update 2012. J
Allergy Clin Immunol 131, 295-299.e1–27 (2013).
25. Domínguez,
O., Plaza, A. M. & Alvaro, M. Relationship Between Atopic Dermatitis and
Food Allergy. Curr Pediatr Rev 16, 115–122 (2020).
26. Blicharz,
L., Rudnicka, L. & Samochocki, Z. Staphylococcus aureus: an underestimated
factor in the pathogenesis of atopic dermatitis? Postepy Dermatol Alergol
36, 11–17 (2019).
27. Miyano,
T., Irvine, A. D. & Tanaka, R. J. Model-Based Meta-Analysis to Optimize
Staphylococcus aureus‒Targeted Therapies for Atopic Dermatitis. JID
Innov 2, 100110 (2022).
28. Vera-Kellet,
C. & Hasbún, C. Eczema herpeticum: A medical emergency in patients with
atopic dermatitis. IDCases 19, e00663 (2020).
29. Mathes,
E. F. et al. ‘Eczema coxsackium’ and unusual cutaneous findings in an
enterovirus outbreak. Pediatrics 132, e149-157 (2013).
30. Achdout,
H. et al. Induction, treatment and prevention of eczema vaccinatum in
atopic dermatitis mouse models. Vaccine 35, 4245–4254 (2017).
31. Flanagan,
S., Schick, A., Lewis, T. P., Chu Tater, K. & Rishniw, M. A survey of
primary care practitioners’ referral habits and recommendations of
allergen-specific immunotherapy for canine and feline patients with atopic
dermatitis. Vet Dermatol 32, 106-e21 (2021).
32. Khorsand,
K. & Brandling-Bennett, H. A. Deficiencies in Dermatologic Training in
Pediatric Residency: Perspective of Pediatric Residency Program Directors. Pediatric
Dermatology 32, 819–824 (2015).
33. Sidbury,
R. et al. Guidelines of care for the management of atopic dermatitis:
Section 4. Prevention of disease flares and use of adjunctive therapies and
approaches. Journal of the American Academy of Dermatology 71,
1218–1233 (2014).
34. Tollefson,
M. M. et al. Atopic Dermatitis: Skin-Directed Management. Pediatrics
134, e1735–e1744 (2014).
35. Grillo,
M., Gassner, L., Marshman, G., Dunn, S. & Hudson, P. Pediatric atopic
eczema: the impact of an educational intervention. Pediatric dermatology
23, (2006).
36. Drislane,
C. & Irvine, A. D. The role of filaggrin in atopic dermatitis and allergic
disease. Ann Allergy Asthma Immunol 124, 36–43 (2020).
37. Eichenfield,
L. F. et al. Guidelines of care for the management of atopic dermatitis:
Section 2. Management and treatment of atopic dermatitis with topical
therapies. Journal of the American Academy of Dermatology 71,
116–132 (2014).
38. Chiang,
C. & Eichenfield, L. F. Quantitative assessment of combination bathing and
moisturizing regimens on skin hydration in atopic dermatitis. Pediatr
Dermatol 26, 273–278 (2009).
39. Katoh,
N. et al. Japanese guidelines for atopic dermatitis 2020. Allergol
Int 69, 356–369 (2020).
40. Dhadwal,
G. et al. Approach to the Assessment and Management of Adult Patients
With Atopic Dermatitis: A Consensus Document. Section IV: Treatment Options for
the Management of Atopic Dermatitis. J Cutan Med Surg 22, 21S-29S
(2018).
41. Draelos,
Z. D. The science behind skin care: Moisturizers. J Cosmet Dermatol 17,
138–144 (2018).
42. Lowe,
A. J. et al. A randomized trial of a barrier lipid replacement strategy
for the prevention of atopic dermatitis and allergic sensitization: the PEBBLES
pilot study. Br J Dermatol 178, e19–e21 (2018).
43. Fleming,
P., Yang, Y. B., Lynde, C., O’Neill, B. & Lee, K. O. Diagnosis and
Management of Atopic Dermatitis for Primary Care Providers. J Am Board Fam
Med 33, 626–635 (2020).
44. Breneman,
D. L., Hanifin, J. M., Berge, C. A., Keswick, B. H. & Neumann, P. B. The
effect of antibacterial soap with 1.5% triclocarban on Staphylococcus aureus in
patients with atopic dermatitis. Cutis 66, 296–300 (2000).
45. Fletcher,
M. J. et al. Improving primary care management of asthma: do we know
what really works? NPJ Prim Care Respir Med 30, 29 (2020).
46. Rea, C.
J. et al. A Randomized Controlled Trial of an Eczema Care Plan. Academic
Pediatrics 18, 789–796 (2018).
47. Ference,
J. D. & Last, A. R. Choosing topical corticosteroids. Am Fam Physician
79, 135–140 (2009).
48. Giannotti,
B. Current treatment guidelines for topical corticosteroids. Drugs 36
Suppl 5, 9–14 (1988).
49. Stacey,
S. K. & McEleney, M. Topical Corticosteroids: Choice and Application. Am
Fam Physician 103, 337–343 (2021).
50. Blauvelt,
A. et al. Long-term management of moderate-to-severe atopic dermatitis
with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a
1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet
389, 2287–2303 (2017).
51. Freeze,
M. E., Balogh, E. A., Cline, A., Feldman, S. R. & Fleischer, A. B.
Comparing prescribing patterns for topical corticosteroids based on their FDA
indication by age. Pediatr Dermatol 38, 115–118 (2021).
52. Siegfried,
E. C., Jaworski, J. C., Kaiser, J. D. & Hebert, A. A. Systematic review of
published trials: long-term safety of topical corticosteroids and topical
calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC
Pediatr 16, 75 (2016).
53. Axon,
E. et al. Safety of topical corticosteroids in atopic eczema: an
umbrella review. BMJ Open 11, e046476 (2021).
54. Arabkhazaeli,
A. et al. Patterns of topical corticosteroids prescriptions in children
with asthma. Pediatr Dermatol 35, 378–383 (2018).
55. Fishbein,
A. B., Silverberg, J. I., Wilson, E. J. & Ong, P. Y. Update on Atopic
Dermatitis: Diagnosis, Severity Assessment, and Treatment Selection. J
Allergy Clin Immunol Pract 8, 91–101 (2020).
56. Hengge,
U. R., Ruzicka, T., Schwartz, R. A. & Cork, M. J. Adverse effects of
topical glucocorticosteroids. J Am Acad Dermatol 54, 1–15; quiz
16–18 (2006).
57. Stoppoloni,
G., Prisco, F., Santinelli, R., Sicuranza, G. & Giordano, C. Potential
hazards of topical steroid therapy. Am J Dis Child 137, 1130–1131
(1983).
58. McAleer,
M. A. et al. Topical corticosteroids normalize both skin and systemic
inflammatory markers in infant atopic dermatitis. Br J Dermatol 185,
153–163 (2021).
59. Birnie,
A. J., Bath-Hextall, F. J., Ravenscroft, J. C. & Williams, H. C.
Interventions to reduce Staphylococcus aureus in the management of atopic
eczema. Cochrane Database Syst Rev CD003871 (2008)
doi:10.1002/14651858.CD003871.pub2.
60. Huang,
J. T., Abrams, M., Tlougan, B., Rademaker, A. & Paller, A. S. Treatment of
Staphylococcus aureus colonization in atopic dermatitis decreases disease
severity. Pediatrics 123, e808-814 (2009).
61. Castillo,
C. R. et al. Assessing the effects of common topical exposures on skin
bacteria associated with atopic dermatitis. Skin Health and Disease 1,
(2021).
62. Nakatsuji,
T. et al. Antimicrobials from human skin commensal bacteria protect
against Staphylococcus aureus and are deficient in atopic dermatitis. Sci
Transl Med 9, eaah4680 (2017).
63. Edslev,
S., Agner, T. & Andersen, P. Skin Microbiome in Atopic Dermatitis. Acta
Derm Venereol 100, adv00164 (2020).
64. Lai, Y.
et al. Commensal bacteria regulate Toll-like receptor 3-dependent
inflammation after skin injury. Nat Med 15, 1377–1382 (2009).



Comments
Post a Comment